K2cr2o7 H2o Estudiar
Potassium dichromate is usually prepared by the reaction of potassium chloride on sodium dichromate. Alternatively, it can be also obtained from potassium chromate by roasting chromite ore with potassium hydroxide. It is soluble in water and in the dissolution process it ionizes: K 2 Cr 2 O 7 → 2 K + + Cr 2O2− 7 Cr 2O2− 7 + H 2 O ⇌ 2 CrO2−
Reagent Friday Chromic Acid, H2CrO4 Master Organic Chemistry
Reactants: K2Cr2O7 - Potassium dichromate (VI) Other names: Potassium dichromate , Chromium potassium oxide , Dipotassium dichromium heptaoxide. show more Appearance: Red-orange crystalline solid ; Orange-to-red crystals H2O2 - Hydrogen peroxide Other names: Dioxidane , Oxidanyl , Perhydroxic acid. show more
K2Cr2O7 Reaction With H2So4 Carbonyl Compounds chem l help you? K2cr2o7 + h2so4 + c3h7oh
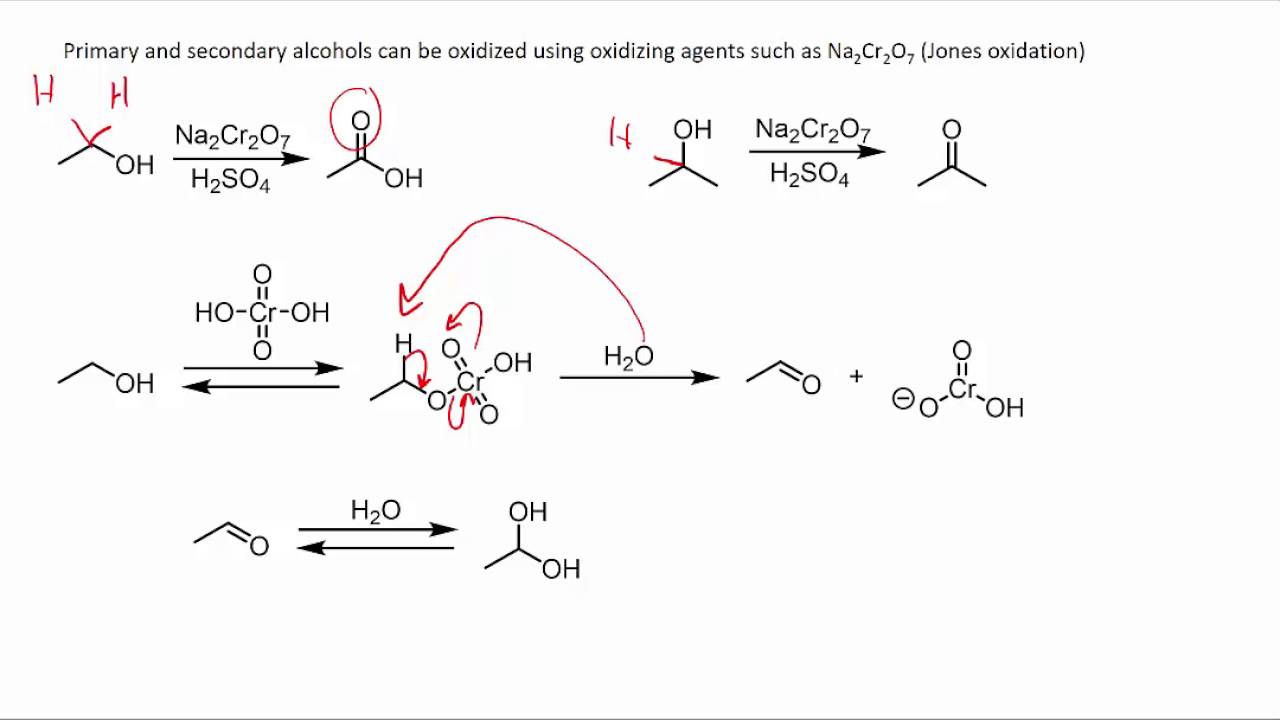
The alcohol is heated under reflux with an excess of the oxidizing agent. When the reaction is complete, the carboxylic acid is distilled off. The full equation for the oxidation of ethanol to ethanoic acid is as follows: 3CH3CH2OH + 2Cr2O2−7 + 16H+ → 3CH3COOH + 4Cr3+ + 11H2O (3) (3) 3 C H 3 C H 2 O H + 2 C r 2 O 7 2 − + 16 H + → 3 C H.
Solved Draw the major organic product of the reaction shown.
Balanced Chemical Equation 303 K 2 Cr 2 O 7 + -5 H 202 + 1212 H 2 SO 4 → 303 K 2 SO 4 + 303 Cr 2 (SO 4) 3 + 707 H 2 O + 707 O 2 Warning: Negative coefficients mean that you should move the corresponding compounds to the opposite side of the reaction.

Preparation of K2Cr2O7 YouTube
The reaction of it is. K2Cr2O7 + 2KOH → 2K2CrO4 + H2O (here K2Cr2O7 is orange and K2CrO4 is yellow). The reaction is K2Cr2O7 + 4 dil.H2SO4 → K2SO4 + Cr2 (SO4)3 + 4H2O + 3(O) Uses of Potassium Dichromate. Potassium dichromate is used in a large amount in the leather industry. The chrome tanning process involves K2Cr2O7.

Chem Expt 3 Reacn. of NaCl & K2Cr2O7 (+ H2SO4) Potassium dichromate, Vapor
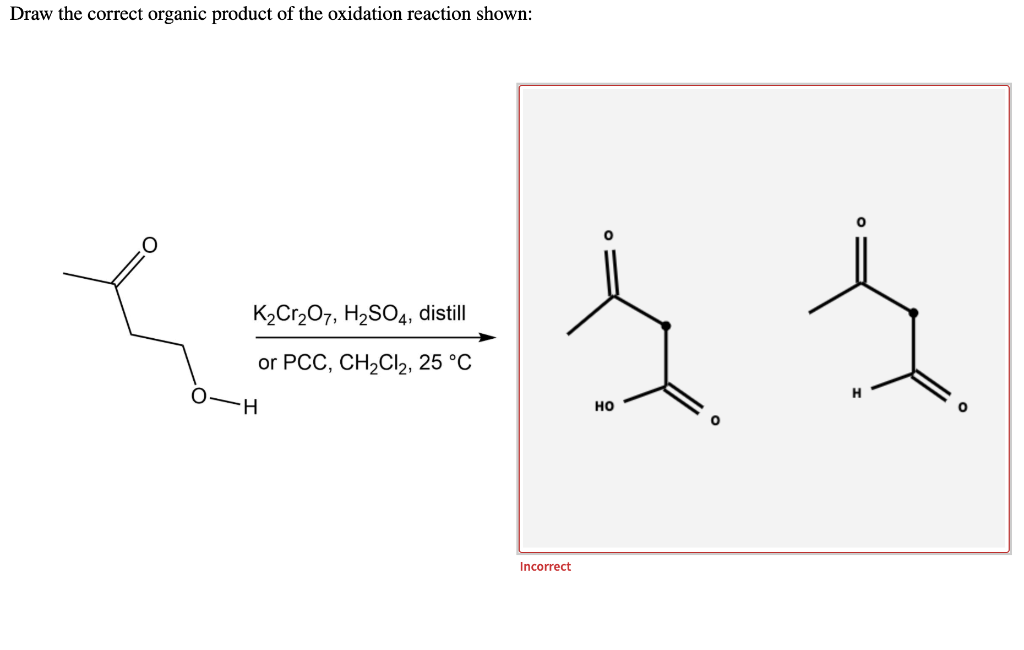
Chemistry Chemistry questions and answers Draw the correct organic product of the oxidation reaction shown: H K2Cr2O7, H2SO4, distill or PCC, CH2Cl2, 25 °C This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

CH3CH2OH+H2SO4+K2Cr2O7=CH3COOH+Cr2(SO4)3+K2SO4+H2O balance the chemical equation by algebraic
10 But I thought why not SOX2 S O X 2 While the reaction is stechiometrically correct, it is hard to perform. In excess of HX2S H X 2 S sulfur will be produced. In excess of oxidant sulfate will be produced. And exact balance is impossible to achieve. That said, SOX2 S O X 2 reacts with HX2S H X 2 S finally producing sulfur.
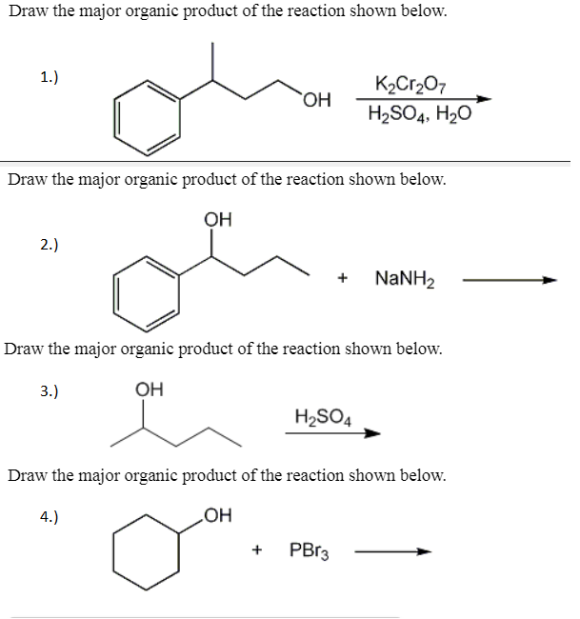
Draw the major organic product of the reaction shown below. K2Cr2O7 H2SO4, H2O WizEdu
Types of Redox Reactions Question In the given reaction, K2Cr2O7 +XH 2SO4 +Y SO2 → K2SO4 +Cr2(SO4)3 +ZH 2O. Find X, Y and Z. Solution Verified by Toppr SO2 +2H 2O (SO4)2− +4H + +2e−……(1) (Cr2O7)2− +14H + +6e− 2G3+ +7H 2O…(2) Multiplying (1) by 3 and adding (2), 3SO2 +(Cr2O7)2− +2H + 3(SO4)2− +2Cr3+ +H 2O Completing the reaction,
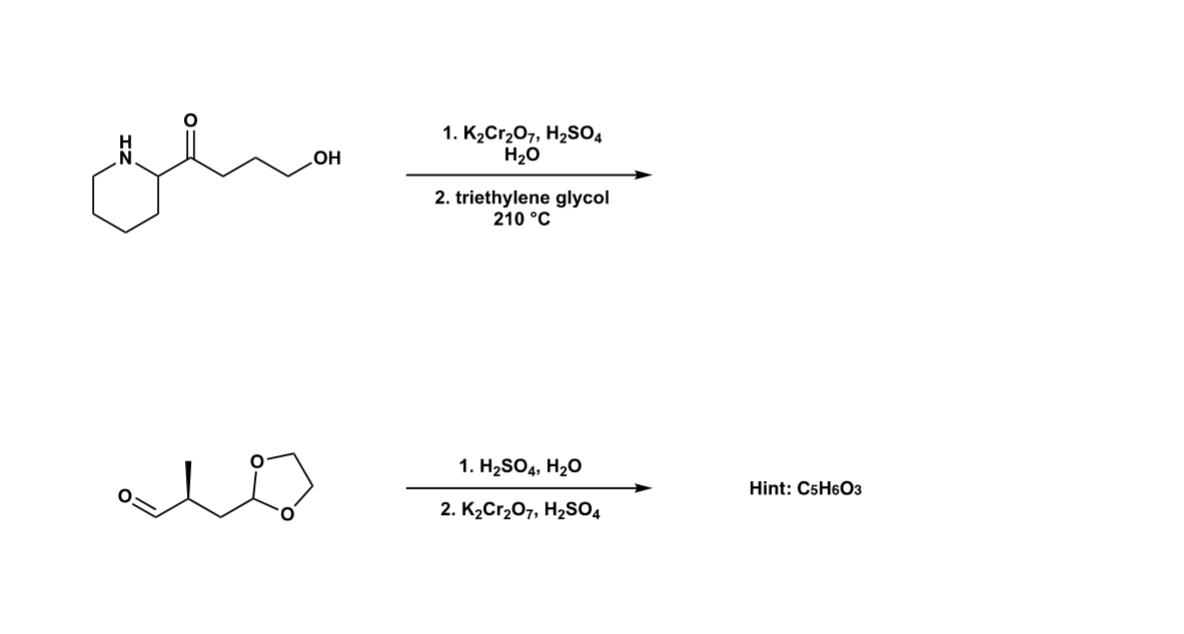
Solved 1. K2Cr2O7, H2SO4 H2O OH 2. triethylene glycol 210 °C
Balanced Chemical Equation K 2 Cr 2 O 7 + -1 H 2 O 2 + 4 H 2 SO 4 → Cr 2 (SO 4) 3 + 3 H 2 O + O 2 + K 2 SO 4 Warning: Negative coefficients mean that you should move the corresponding compounds to the opposite side of the reaction. Warning: One of the compounds in K2Cr2O7 + H2O2 + H2SO4 = Cr2 (SO4)3 + H2O + O2 + K2SO4 is unrecognized.

😊 H2o2 k2cr2o7. Redox uncertainty Cr2O7 + H2SO4 + H2O2? chemhelp. 20190226
The reaction between H2SO4 and K2Cr2O7 is commonly used in organic chemistry as a test for the presence of alcohols, as it can oxidize alcohols to form aldehydes or ketones. Solubility of K2SO4 in water Potassium sulfate (K2SO4) is a compound that readily dissolves in water.

Potassium dichromate (K2Cr2O7) react with sodium sulfite and sulfuric acid K2Cr2O7+H2SO4
Q. In the chemical reaction, K2Cr2O7+xH2SO4+ySO2→K2SO4+Cr2(SO4)3+zH2O the value of x+y+z: Q. In the chemical reaction, K2Cr2O7+XH2SO4+Y SO2→K2SO4+Cr(SO4)3+zH2O, X, Y and Z are respectively, Q. For the oxidation-reduction reaction; K2Cr2O7+XH2SO4+Y SO2→K2SO4+Cr2(SO4)3+ZH2O The values X, Y and Z are: Q.
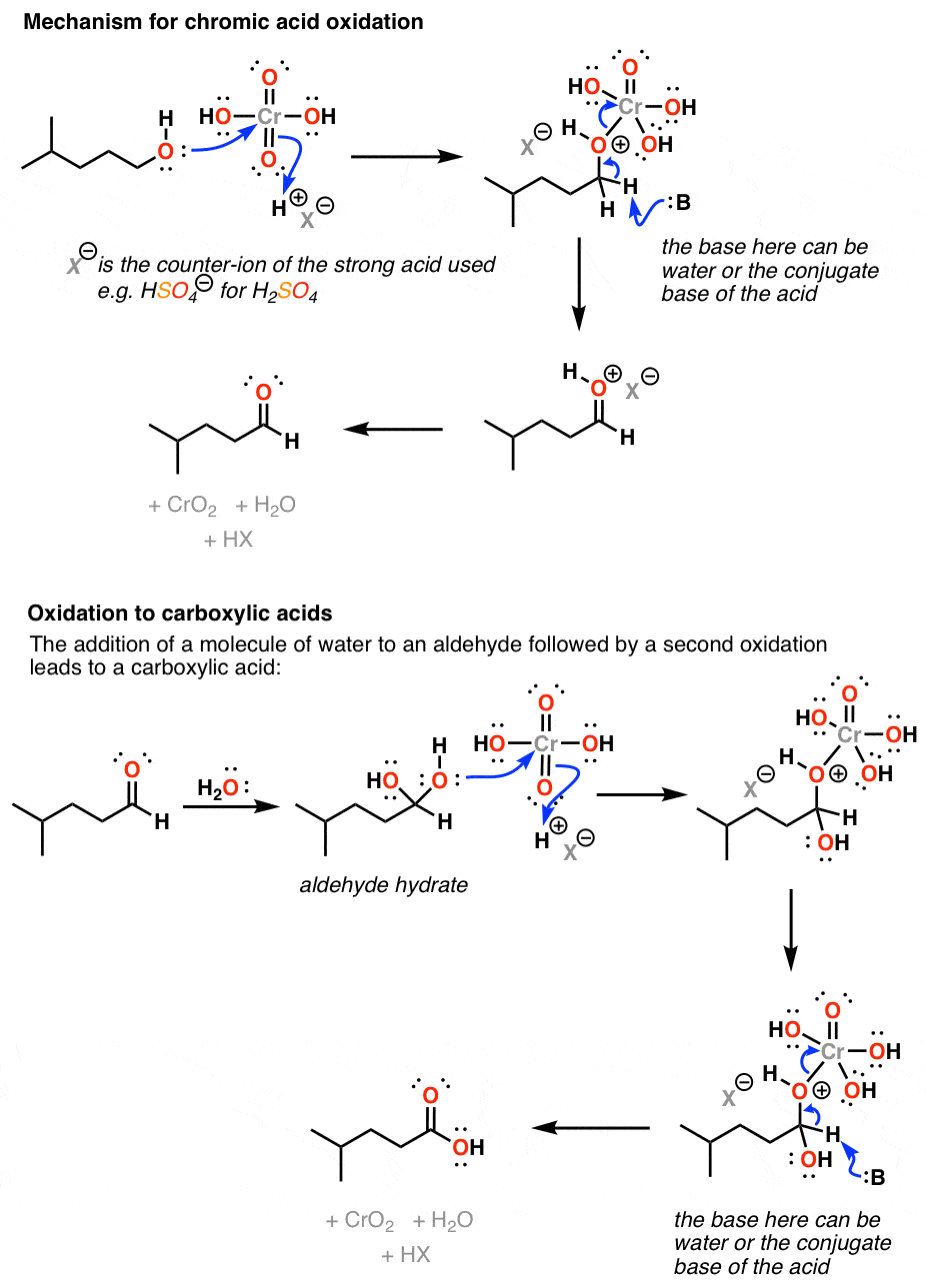
Chromic acid (H2CrO4) as an oxidant in organic chemistry — Master Organic Chemistry
Balanced Chemical Equation 0 K 2 Cr 2 O 7 + 3 H 2 S + H 2 SO 4 → 0 Cr 2 (SO 4) 3 + 0 K 2 SO 4 + 4 S + 4 H 2 O Warning: Some compounds do not play a role in the reaction and have 0 coefficients. Make sure you have entered the equation properly. Warning: One of the compounds in K2Cr2O7 + H2S + H2SO4 = Cr2 (SO4)3 + K2SO4 + S + H2O is unrecognized.
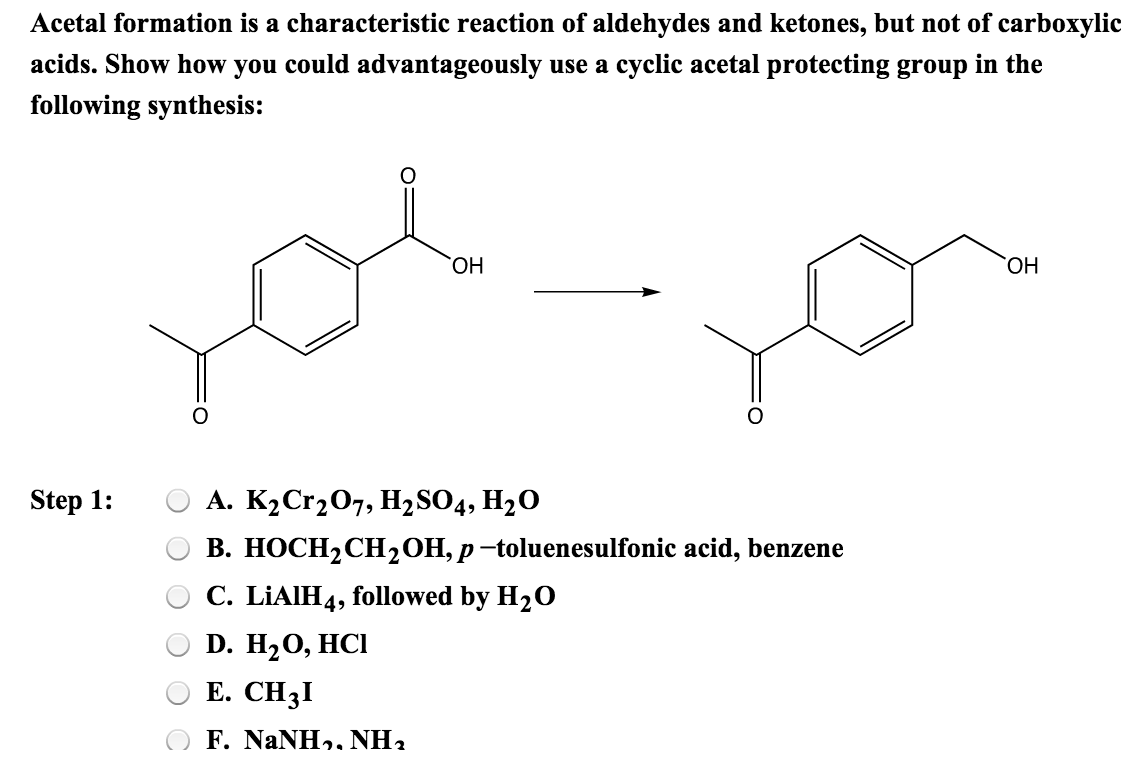
Solved Acetal Formation Is A Characteristic Reaction Of A...
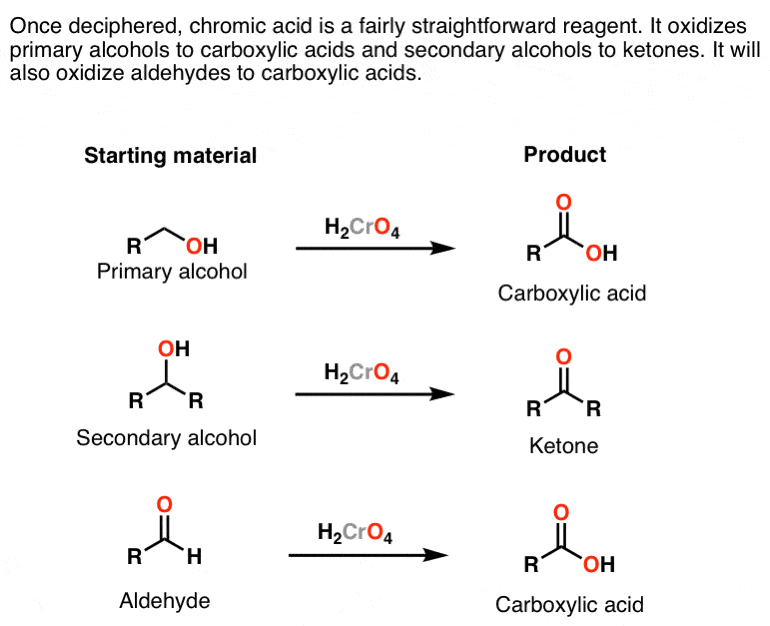
By using jones reagent , we get RCHO group ie , an aldehyde. Jones reagent is a relatively mild oxidising agent. Only a strong oxidising ahent such as chromic acid (H2CrO4) could oxidise an alcohol to carboxylic acid. The oxidising order is as follows -. alkanes -> alcohols -> aldehydes -> carboxylic group. 1 comment.
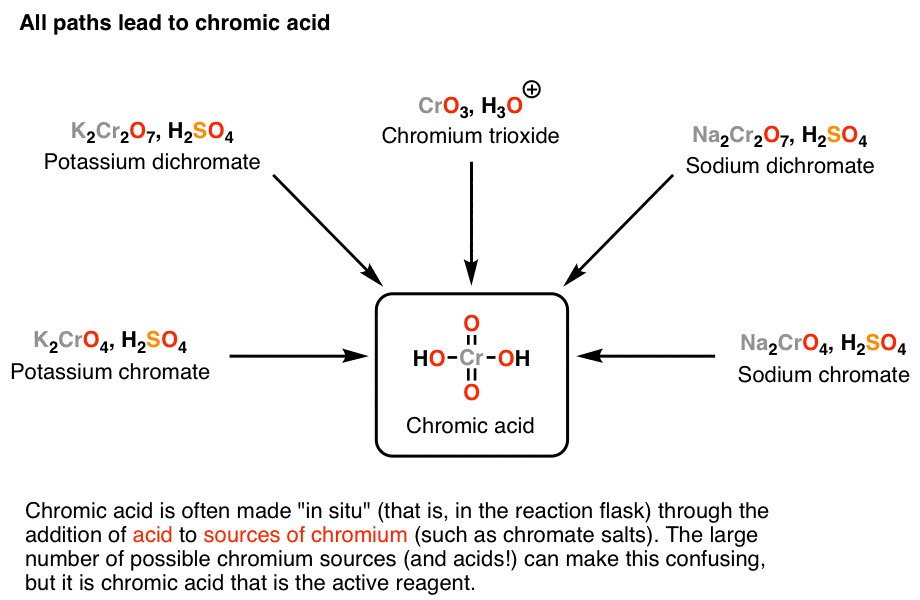
Reagent Friday Chromic Acid, H2CrO4 Master Organic Chemistry
Explanation: The balanced equation is K2Cr2O7 + 3SO2 +H2SO4 → Cr2(SO4)3 +K2SO4 +H2O I think you are referring to the ion-electron method or the half-reaction method. Step 1. Write the skeleton equation The molecular equation is K2Cr2O7 + H2SO4 +SO2 → K2SO4 + Cr2(SO4)3 +H2O

K2Cr2O7 + H2So4 + Feso4 / Используя метод инноэлектронного баланса,расставьте коэф Don't
This video is the practical demonstration of the reaction of Acidified Potassium dichromate (k2Cr2O7+H2SO4) with Hydrogen peroxide (H2O2).Precipitation and d.

😊 H2o2 k2cr2o7. Redox uncertainty Cr2O7 + H2SO4 + H2O2? chemhelp. 20190226
Balanced Chemical Equation 2 K 2 Cr 2 O 7 + 8 H 2 SO 4 → 2 K 2 SO 4 + 2 Cr 2 (SO 4) 3 + 8 H 2 O + 3 O 2 Warning: One of the compounds in K2Cr2O7 + H2SO4 = K2SO4 + Cr2 (SO4)3 + H2O + O2 is unrecognized. Verify 'Cr2 (SO4)3' is entered correctly. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation.